M.K. Maid1*
, R.R. Deshmukh2
1*Department of CS and IT, Dr. B. A. M. U, Aurangabad, India
2Department of CS and IT, Dr. B. A. M. U, Aurangabad, India
*Corresponding Author: mm915monali@gmail.com
Available online at: www.ijcseonline.org
Abstract— Remote Sensing has wide range of applications in many different fields. Remote Sensing has been found to be a valuable tool in evaluation, monitoring, and management of land, water and crop resources. The applications of remote sensing techniques in the field of agriculture are wide and varied ranging from crop identification, detection of disease on different crops & predicting grain yield of crops. Many remote sensing applications are devoted to the agricultural sector. The selected applications are put in the context of the global challenges the agricultural sector is facing: minimizing the environmental impact, while increasing production and productivity. The application of remote sensing in agriculture typically involves measuring reflectance of electromagnetic radiation in the visible (390 to 770 nm), near-infrared (NIR, 770 to 1,300 nm), or middle-infrared (1,300 to 2,500 nm) ranges using spectrometers. This paper reviews the concept of hyperspectral remote sensing, use of remote sensing in terms of agriculture field, study of diseased wheat leaves using hyperspectral remote sensing.
Keywords—Remote Sensing, Wheat Leaf Rust, Vegetation Indices, ASD Fieldspec4 Spectroradiometer.
I. INTRODUCTION
Remote sensing refers to the activities of recording/observing/perceiving (sensing) objects or events at far away (remote) places. Remote sensing is a sub-field of geography. In modern usage, the term generally refers to the use of aerial sensor technologies to detect and classify objects on Earth (both on the surface, and in the atmosphere and oceans) by means of propagated signals (e.g. electromagnetic radiation) [1]. The electromagnetic radiation is normally used as an information carrier in remote sensing. The reflection of that energy by earth surface materials is then measured to produce an image of the area sensed. Generally, Remote sensing can be done on two types of data namely imagery and non imagery. It can be done using different kinds of remote sensing devices like ASD fieldspec Spectroradiometer. Remote sensing have wide range of applications in various fields, among which Agriculture plays important role in our day to day life as not only in india but in many countries agriculture is their primary source of income and all human beings, animals and many industries are dependent on agriculture field. agriculture plays key macroeconomic roles in the
industrialization of developing countries by relieving saving, aggregate demand, fiscal, and foreign exchange constraints on the industrial sector [2].
In agriculture field winter wheat is one of the highest yielding crops on the farm [3]. Different climatic factors and disease symptoms affects the plant growth and it directly results in yield of crop. Rust are among the most important
fungal diseases of wheat worldwide [4]. There are three types of rust diseases in wheat crop: Strip Rust, Leaf Rust, Stem Rust.
Wheat rusts are caused by three related fungi [5]:
• Stripe rust is caused by Puccinia striiformis f. sp. tritici.
• Leaf rust is caused by Puccinia triticina.
• Stem rust is caused by Puccinia graminis f. sp. tritici.
This paper reviews the study of wheat leaf rust (WLR) disease using hyperspectral analysis, different vegetation indices and spectral signatures can be used to estimate the features of diseased and healthy crop. In this review paper ASD Fieldspec4 Spectroradiometer is used for data collection of diseased wheat leaves and healthy wheat leaves. Using different vegetation indices (VIs) biophysical and biochemical properties of crop can be estimated.
II. BASICS OF REMOTE SENSING
Hyperspectral remote sensing is used for over 100 years for analysis of various objects and their chemical as well as biological composition. But hyperspectral sensor offers an alternate and nondestructive technique for analysis of
physical and chemical properties of material. Remote sensing of vegetation is mainly performed by obtaining the electromagnetic wave reflectance information from canopies using passive sensors. It is well known that the reflectance of
light spectra from plants changes with plant type, water content within tissues, and other intrinsic factors [6].
The reflectance from vegetation to the electromagnetic spectrum (spectral reflectance or emission characteristics of vegetation) is determined by chemical and morphological characteristics of the surface of organs or leaves [7].
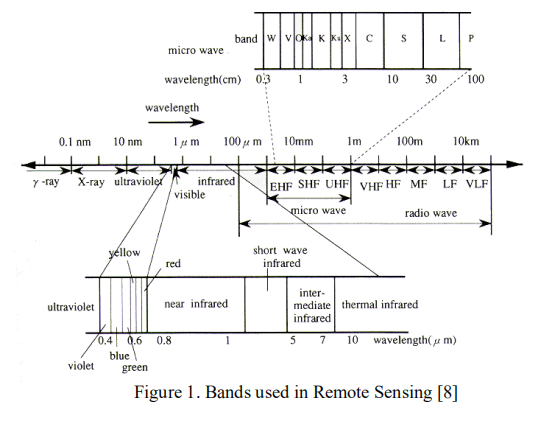
The main applications for remote sensing of vegetation are based on the following light spectra: (i) the ultraviolet region (UV), which goes from 10 to 380 nm; (ii) the visible spectra, which are composed of the blue (450–495 nm), green (495−570 nm), and red (620–750 nm) wavelength regions; and (iii) the near and mid infrared band (850–1700 nm)[9,10].
III. HYPERSPECTRAL REMOTE SENSING IN AGRICULTURE
Spectral data at the leaf and canopy scales have been utilized to improve the plant disease detection techniques from remotely sensed observations [11,12], where the visible and infrared regions are more sensitive to disease development [13]. The measured spectra can be utilized to early detection of fungus disease. Moreover, the optimized narrow bands vegetation indices were employed to discriminate various disease of wheat [14].
III.I Wheat Leaf Rust (WLR) Disease
The wheat rust is an important crop disease which has three types, i.e., wheat yellow rust (WYR), wheat leaf rust (WLR),and wheat stem rust [15].

WYR disease is identified by a single symptom which occurs as a narrow yellow stripes parallel to nervures on the leaf, whereas WLR disease is caused by the Puccinia triticina fungus and illustrates numerous symptoms simultaneously in various parts of an infected leaf [16]. The WLR symptoms vary from leaf to leaf but it presents a yellow color earlier, then its changes to orange and dark brown. Finally, the disease symptom ends with the dry leaf [17].

The effect of a disease on the pigments and structure of a plant and the change in their spectral responses enable spectroradiometry and remote sensing techniques to detect plant disease effectively [18].
Crop disease can cause significant yield loss and reduction of grain quality, which have a negative impact to food security around the world [19].
IV. EXPERIMENTAL SETUP
IV.I Data Collection
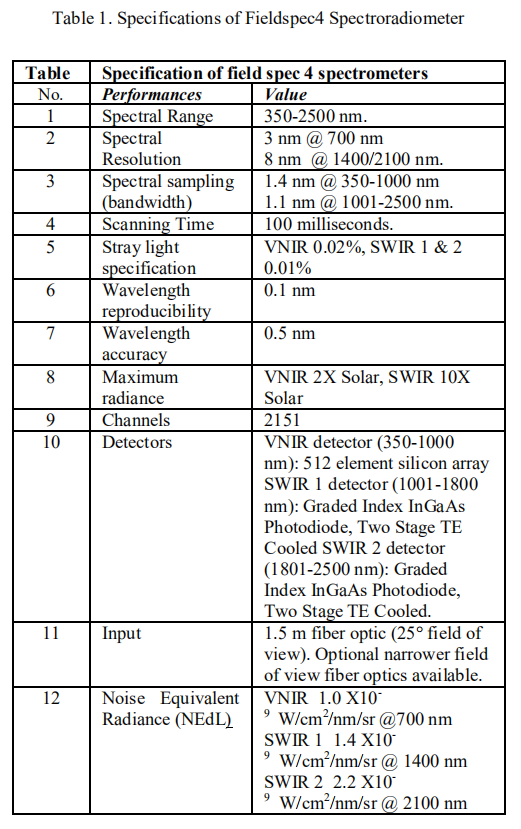
Field spec 4 spectrometer (Analytical spectral device, ASD Co. USA) shown in following figure having parameter details in Table 1. Spectrum data export in ASCII text, then it can analyze spectrum data with different software like ASD View Spec Pro. Unscramble and MATLAB/ Octave [20].

V. VEGETATION INDICES FOR ESTIMATION OF WLRSYMPTOMS
Spectral data at different scales including leaf, canopy and landscape-level have been widely used to improve precision [21-24]. In recent years, researchers have studied various spectral vegetation indices (SVIs) to detect different
vegetation diseases [24-26]. Efficient use of spectral data in detection of plant disease depends on the application. The spectral regions from 400 to 700 and 700 to 1100 are mainly influenced by leaf composition of pigments, structure, and
water content [27]. The effect of a disease on the pigments and structure of a plant and the change in their spectral responses enable spectroradiometry and remote sensing techniques to detect plant disease effectively [28]. There are
indices derived from reflectance values at several wavelengths that are able to detect and quantify the leaf content substances such as chlorophyll, anthocyanin, and water [29,30].
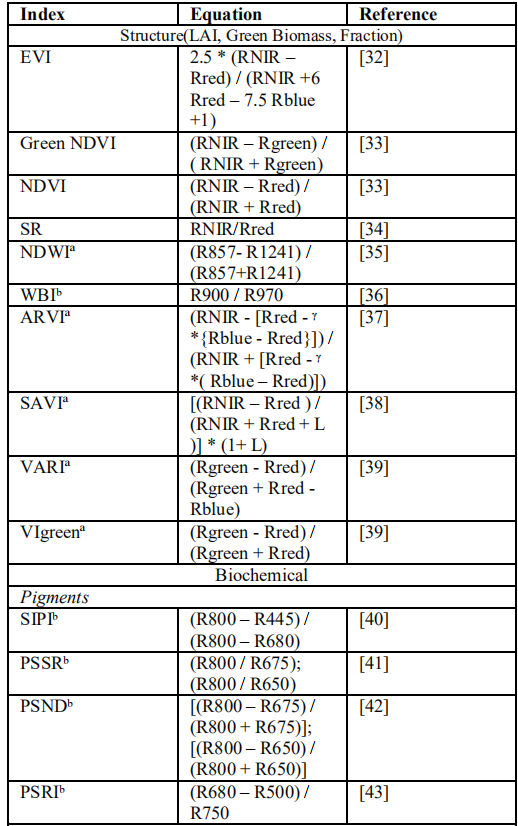
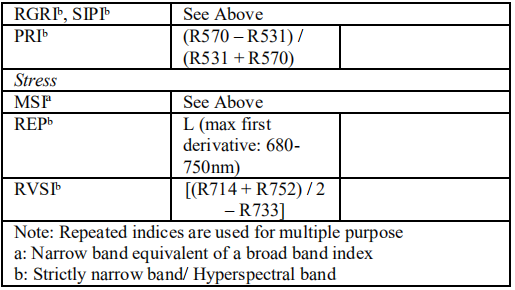
By using different types of vegetation indices estimation of biochemical and biophysical properties of crops is possible. Vegetation indices that are used by many researchers have shown in following table [31].
Table 2. Different Vegetation Indices

VI. CONCLUSION
As Remote Sensing technology growing rapidly in technological era and hyperspectral Remote sensing has wide number of applications not only in agriculture field but also in different industries which are dependent on agricultural area. With the help of different spectral characteristics like spectral signatures, vegetation indices, reflectance spectra we can use it for discrimination of crops. It can be used to study the severity of disease in crops, estimating the grain yield of crops, analysis and growth modulation of crop.
ACKNOWLEDGMENT
This work is supported by Dept. of Computer Science and Information Technology under the funds for Infrastructure under science and Technology (DST-FIST) with sanction no. SR/FST/ETI- 340/2013 to Dept. of Computer Science and Information Technology, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, Maharashtra, India. The authors would like to thank Department and University Authorities for providing the infrastructure and necessary
support for carrying out the research.
REFERENCES
[1] A. Chitradevi, S. Vijayalakshmi, “Random Forest for Multitemporal and Multiscale Classification of Remote Sensing Satellite Imagery”, International Journal of Computer Sciences and Engineering, Vol. 4, Issue.2, pp.59-65, 2016.
[2] D. Souza, “Growth Complementarity Between Agriculture and Industry: Evidence from a Panel of Developing Countries”, 2014.
[3] G. Boyle, “The Winter Wheat Guide”, Teagasc, pp. 21-40, 2016.
[4] S. N. Wegulo, “Rust Diseases of Wheat”, NebGuide, 2012.
[5] S. Markell, G. Milus, R. Cartwright, J. Hedge, “Rust Diseases of Wheat”, Agriculture and natural resources.
[6] L. Chang, S. Peng-Sen, and Liu Shi-Rong, “A review of plant spectral reflectance response to water physiological changes,” Chinese Journal of Plant Ecology, vol. 40, no. 1, pp. 80–91, 2016.
[7] C. Zhang and J. M. Kovacs, “The application of small unmanned aerial systems for precision agriculture: a review,” Precision Agriculture, vol. 13, no. 6, pp. 693–712, 2012.
[8] J. B Campbell, “Introduction to Remote Sensing”, Taylor and Francis, London, 1996.
[9] H. R. Bin Abdul Rahim, M. Q. Bin Lokman, S. W. Harun, “Applied light-side coupling with optimized spiral-patterned zinc oxide nanorod coatings for multiple optical channel alcohol vapor sensing,” Journal of Nanophotonics, vol. 10, no. 3, Article ID 036009, 2016.
[10] B. A. Cruden, D. Prabhu, and R. Martinez, “Absolute radiation measurement in venus and mars entry conditions,” Journal of Spacecraft and Rockets, vol. 49, no. 6, pp. 1069–1079, 2012.
[11] S. Sankaran, A. Mishra, R. Ehsani, and C. Davis, “A review of advanced techniques for detecting plant diseases,” Comput. Electron. Agriculture, vol. 72, no. 1, pp. 1–13, 2010.
[12] C. Buschmann and E. Nagel, “In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation,” Int. J. Remote Sens, vol. 14, no. 4, pp. 711–722, 1993.
[13] N. K. Poona and R. Ismail, “Using Boruta-selected spectroscopic wavebands for the asymptomatic detection of Fusarium circinatum stress,” IEEE J. Select. Topics Appl. Earth Observations Remote Sens., vol. 7, no. 9, pp. 3764–3772, 2014.
[14] W. Huang, “New optimized spectral indices for identifying and monitoring winter wheat diseases,” IEEE J. Select. Topics Appl. Earth Observations Remote Sens., vol. 7, no. 6, pp. 2516–2524, 2014.
[15] M. D. Bolton, J. A. Kolmer, and D. F. Garvin, “Wheat leaf rust caused by Puccinia triticina,” Molecular Plant Pathology, vol. 9, no. 5, pp. 563–575, 2008.
[16] C. Robert, M.-O. Bancal, B. Ney, and C. Lannou, “Wheat leaf photosynthesis loss due to leaf rust, with respect to lesion development and leaf nitrogen status,” New Phytologist, vol. 165, no. 1, pp. 227–241, 2005.
[17] D. Ashourloo, H. Aghighi, A. A. Matkan, M. R. Mobasheri, and A. M. Rad, “An Investigation Into Machine Learning Regression Techniques For The Leaf Rust Disease Detection Using Hyperspectral Measurement”, IEEE journal of selected topics in applied earth observations and remote sensing, vol. 9, pp. 4344 – 4351, 2016.
[18] J.C. Zhang, R.L. Pu, J.H.Wang, W.J. Huang, L.Yuan, J.H. Luo, “Detecting powdery mildew of winter wheat using leaf level hyperspectral measurements”, Comput. Electron. Agric, pp. 13–23, 2012.
[19] R. N. Strange, P. R. Scott, “Plant Disease: A threat to global food security”, Annual reviews phytopathol, vol. 43, pp. 83-116, 2005.
[20] R. M. Misal, R. R. Deshmukh, “Application of Near-Infrared Spectrometer in Agro-Food Analysis: A Review”, International Journal of Computer Applications, Vol. 141 No.7, pp. 0975 – 8887, 2016.
[21] H.D Roelofsen, P. M. van Bodegom, L. Kooistra, , J. P.M. Witte, “Trait estimation in herbaceous plant assemblages from in situ canopy spectra” Remote Sens., Vol. 5, pp. 6323–6345, 2013.
[22] S. Delalieux, A. Auwerkerken, V.W. Verstraeten, B. Somers, R.Valcke, S.Lhermitte, J. Keulemanss, P. Coppin, “Hyperspectral reflectance and fluorescence imaging to detect scab induced stress in Apple leaves”, Remote Sens, Vol. 1, pp. 858–874, 2009.
[23] U. Steiner, K. Bürling, E.C. Oerke, “Sensor use in plant protection”, Gesunde Pflanz, Vol. 60, pp. 131–141, 2008.
[24] J.C. Zhang, R.L. Pu, J.H.Wang, W.J. Huang, L.Yuan, J .Wang, “Using in-situ hyperspectral data for detecting and discriminating yellow rust disease from nutrient stresse”,Field Crops Res., Vol. 134, pp.165–174,2012.
[25] C.Hillnhütter, A.K. Mahlein, R.A. Sikora, E.C. Oerke, “Remote sensing to detect plant stress induced by Heterodera schachtii and Rhizoctonia solani in sugar beet fields”, Field Crops Res., Vol. 122, pp. 70–77, 2011.
[26] D. Moshou, C. Bravo, J. West, S. Wahlen, A. McCartney, H. Ramon, “Automatic detection of ―yellow rust‖ in wheat using reflectance measurements and neural networks”, Comput. Electron. Agric, Vol. 44, pp. 173–188, 2004.
[27] A.K. Mahlein, T. Rumpf, P. Welke, H.W. Dehne, L. Plümer, U. Steiner, E.C. Oerke, “Development of spectral indices for detecting and identifying plant diseases”, Remote Sens. Environ, Vol. 128, pp. 21–30, 2013.
[28] J.C. Zhang, R.L. Pu, J.H.Wang, W.J. Huang, L. Yuan, J.H. Luo, “Detecting powdery mildew of winter wheat using leaf level13–23, 2012.
[29] A.A. Gitelson, Y.J. Kaufman, R. Stark, D. Rundquist, “Novel algorithms for remote estimation of vegetation fraction”, Remote Sens. Environ, Vol.80, pp. 76–87, 2002.
[30] J. Penuelas, F. Baret, I. Filella, “Semiempirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance”, Photosynthetica, Vol. 31, pp. 221–230, 1995.
[31] P. V. Janse, R. R. Deshmukh, “Hyperspectal Remote Sensing for Agriculture: A Review”, International Journal of Computer Applications,Vol.172 No.7, pp. 0975 – 8887, 2017.
[32] A. R. Huete, B. K. Liu, L. Van, “A comparison of vegetation indices over a global set of TM images for EOS-MODIS”, Remote Sensing of Environment, Vol. 59, pp. 440-451, 1997.
[33] J.W. Rouse, R.H. Haas, J.A. Schell, D.W. Deering, “Monitoring vegetation systems in the great plains with ERTS, Third ERTS symposium”, NASA SP-351, NASA Washington, DC, Vol. 1, pp. 309-317, 1973.
[34] C.F. Jorden, “Leaf area index from quality of light on the forest floor”, Ecology, Vol. 50(4), pp. 663-666, 1969.
[35] B. Gao, “NDWI: A normalized difference water index for remote sensing of vegetation liquid water from space”, Remote Sensing of Environment, Vol. 58, pp. 257-266, 1996.
[36] J. Penuelas, J. Pinol, R. Ogaya, I. Lilella, “Estimation of plant water content by the reflectance water index WI (R900/ R970)”, International journal of remote sensing, Vol. 18, pp. 2869-2875, 1997.
[37] Y. J. Kaufman, D. Tanier, “Atmospherically resistant vegetation index (ARVI) for EOS-MODIS”, IEEE Transaction on Geoscience and Remote Sensing, Vol. 30(2), pp. 261-270, 1992.
[38] A.R. Huete, “A soil adjusted vegetation index (SAVI)”, Remote Sensing of Environment, Vol. 71, pp. 158-182, 2000.
[39] A.A. Gitelson, Y. J. Kaufman, R. Stark, D. Rundquist, “Novel algorithm for remote estimation of vegetation fraction”, Remote Sensing of Environment, vol. 80, pp. 76-87, 2002.
[40] J. Penuelas, F. Baret, I. Filella, “Semi empirical indices to assess carotenoids/ chlorophyll a ratio from leaf spectral reflectance”, Photosynthetica, Vol. 31, pp. 221-230, 1995.
[41] G. A. Blackburn, “Spectral indices for estimating photosynthetic pigment concentration: A test using senescent tree leaves”, International journal of remote sensing, Vol. 19, pp. 657-675, 1998.
[42] G. A. Blackburn, “Quantifying chlorophyll and carotenoids from leaf to canopy scale: An evaluation of some hyperspectral approaches”, Remote Sensing of Environment, Vol. 66, pp. 273-285, 1998.
[43] M. N. Merzlyak, A. A. Gitelson, O. B. Chivkunova, Y. Ratikin, “Non�destructive optical detection of pigment changes during leaf senescent and fruit ripening”, Physiologia Plantarum, Vol. 105, pp. 135-141, 1999.
[44] M. S. Kim, “The use of narrow spectral bands for improving remote sensing estimation of fractionally absorbed photosynthetically active radiation (fAPAR)”, Master Thesis, Department of Geography, University of Maryland, College Park, 1994.
[45] C. S. T. Daughtry, C. L. Walthall, M. S. Kim, E. B. de Colstoun, J. E. McMurtrey, “Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance”, Remote Sensing of Environment, Vol. 74, pp. 229-239, 2000.
[46] A. A. Gitelson, G. P. Keydan, M. N. Merzlyak, “Three band model for noninvasive estimation of chlorophyll, carotenoids and anthocyanin contents in higher plant leaves”, Geophysical Research Letters, Vol. 33, L11402, 2006.
[47] A. A. Gitelson, M. N. Merzlyak, O. B. Chivkunova, “Optical properties and non-destructive estimation of anthocyanin content in plant leaves”, Photochemistry and Photobiology, Vol. 74(1), pp. 38-45, 2001.
[48] J. A. Gaman, J. S. Surfus, “Assessing leaf pigment content and activity with a reflectometer”, New Phytologist, Vol. 143, pp. 105-117, 1999.
[49] A. K. Van Den Berg, T. D. Perkins, “Non-destructive estimation of anthocyanin content in autumn auger maple leaves”, Horticultural Science, vol. 40(3), pp. 685-685, 2005.
[50] A. A. Gitelson, Y. Zur, O. B. Chivkunova, M. N. Merzlyak, “Assessing carotenoid content in plant leaves with reflectance spectroscopy, Photochemistry and Photobiology, Vol. 75(3), pp. 272-281, 2002.
[51] A. R. Hunt, B. N. Rock, “Detection of changes in leaf water content using near- and middle-infrared reflectance”, Remote Sensing of Environment, Vol. 30, pp. 43-54, 1989.
[52] B. N. Rock, J. E. Vogelmann, D. L. Williams, A. F. Vogelmann, T. Hoshizaki, “Detection of forest damage”, BioScience, Vol. 36(7), pp. 439-445, 1986.
[53] J. A. Gamon, L. Serrano, J. S. Surfus, “The photochemical reflectance index: An optical indicator of photosynthetic radiation-use efficiency across species, functional types, and nutrient level”, Oecologia, Vol. 112, pp. 492-501, 1997.
[54] D. N. H. Horler, M. Dockray, J. Barber, “The red-edge of plant leaf reflectance”, International journal of remote sensing, Vol. 4, pp. 273-288, 1983.